Anti-inflammatory and Antioxidant Effects on In Vitro Cultured Adventitious Root Extracts to Different Platycodon grandiflorum Flower Colors
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
초록
This study explored the anti-inflammatory and antioxidant activity according to the flowers’ color and the flowers’ shape from in vitro cultured Platycodon grandiflorum (PG) adventitious root extract. The DPPH and ABTS radical scavenging activity showed that the increase was proportional to the extract’s concentration. As a result of measuring the DPPH and ABTS radical scavenging activity, it was confirmed that the component that enhanced antioxidation was contained in the PG adventitious root, and it was presumed that there was not a large change in the scavenging activity according to the flowers’ color and shape. The nitrite scavenging effect was the highest at pH 1.2 in all the tested samples. However, there was no distinct detection of nitrite scavenging effects of the pH range 6.0. After PG extract pretreatment at 50, 100, and 200 μg mL-1, the LPS-treated experimental group significantly inhibited NO production in a dose-dependent manner. The dose-response trends followed quadratic regressions in all the tested PG adventitious root extracts. The PG adventitious root extracts showed a considerable range of influence on cytokine secretion. The adventitious root extracts of PG against the production of inflammatory mediators TNF-α, IL-6 and IL-1β showed significant anti-inflammatory activity.
Keywords:
ABTS, Anti-inflammatory, DPPH, Nitrite Scavenging Activity, Platycodon grandiflorum, RAW 264.7 cellI. INTRODUCTION
The root of Platycodon grandiflorum (PG) that belongs to the Campanulaceae family have been used as a food material and a traditional oriental medicine. In Korea, the PG has been collected and grown wild in the field, but in recent years, the demand for PG has increased, and cultivation has increased in general farms. The PG is a plant that contains protein, lipids, sugars, starch, iron, saponin, inulin, phytosterin, platycodinin, etc. The flower colors are white, purple and pink, and the flower shapes are single petal and double petal flower. The PG flowers are beautifully bloomed and used as a garden, landscaping, and cut flower, and are available native plant that use both above- and below-ground parts. The extracts from the PG root revealed that it contains a wide variety of compounds having health benefits including immunopharmacological effects (Gao et al. 2017). Recently, plant and plant-derived products are treated a part of the healthcare system by applying the bioactive phytochemicals. Medicinal plants are belived to be a potential source for the research of new biologically active compounds. Antioxidants are the compounds that delay the oxidation of other molecules by inhibiting the initiation or propagation of oxidizing chain reactions. Finding new and safe antioxidants from natural sources is great interest for applications in natural antioxidants, functional foods, and neutraceuticals. Phytochemical screening is one of the methods that have been used to explore antioxidant compounds in plants (Do et al. 2014). Antioxidant compounds can scavenge free radicals and increase shelf life by retarding the process of lipid peroxidation, which is one of the major reasons for deterioration of food and pharmaceutical products during processing and storage (Gülçin 2010). Bioactive phytochemicals are increasingly used in the healthcare system. In particular, the screening of medicinal plants with antioxidant and anti-inflammatory activity is a major research goal worldwide. Antioxidant compounds, characterized by the ability to eliminate free radicals, in food have important protective effects on health. Many dietary antioxidant compounds are derived from plant sources, with a wide variety of physical and chemical properties. Furthermore, various plant extracts have be shown to suppress the production of proinflammatory cytokines, such as TNF-α, IL-6, and IL-1β, based on extensive in vitro analyses (Choi et al. 2016). Many medicinal plants such as PG have beneficial sources of anti-inflammatory agents. Accordingly, the research of the anti-inflammatory cytokines and antioxidant activity of PG roots is great attention and crucial factor for human health. In this study, the anti-inflammatory and antioxidant activity of different fractional extracts of PG were evaluated. This experiment was carried out to identify the difference in the anti-inflammatory and antioxidant activity of the PG root according to flower color and flower shape, including the green petal flower newly developed by our research team.
II. MATERIALS AND METHODS
1. Sample preparation
Adventitious root samples of different PG flower colors were provided by the cooperative research laboratory of this study, Woosong Information College. In vitro cultured adventitious root samples were freeze dried and ground to a fine powder. The powder was stored at -20°C until further analyses. The freeze dried powder was immersed in 70% methanol and the filtrate was collected for three times with constant stirring of the mixture at every 24 hr interval of a 72 hr total collection period. The filtrate was then concentrated under reduced pressure at 45°C using a vacuum rotary evaporator (IKA® RV 10 Basic Digital, IKA Co., Germany). The concentrated extract was stored at -20°C until further analysis
2. Determination of DPPH radical scavenging activity
The free radical scavenging activity of PG was measured by DPPH using the method described by Brand-Williams et al. (1995). In brief, 0.1 mM DPPH in methanol was prepared and 0.9 mL of this solution was added to 0.1 mL of GEB fractional extracts at different concentrations. The mixtured solution was allowed to react for 5 min by shaking with vortexing. The reacted sample was stand at room temperature and dark for 30 min, and then the absorbance was measured at 517 nm using a spectrophotometer. The DPPH radical scavenging capability was calculated according to the following equation: DPPH radicals (%) = [(A–B)/A] × 100], where A is the absorbance of the control reaction and B is the absorbance in the present of the sample of PG extract.
3. Determination of ABTS radical cation (ABTS•+)
ABTS•+ assay was measured spectrophotometry by applying Re et al.(1999) previous method. In brief, ABTS was dissolved in water to a 7 mM concentration. ABTS•+ was produced by reacting ABTS stock solution with 2.45 mM potassium persulfate and allowed to stand in the dark at room temperature for 15 hr, followed by dilution to an absorbance of 0.7 at 734 nm. The 50 μL PG sample at different concentration with 0.95 mL of the diluted solution were added, followed by shaking for 10 sec by vortexing. The samples were reacted for 5 min at room temperature and absorbance was read at 734 nm. The ABTS cation radical scavenging capacity was calculated relative to the reactivity of ascorbic acid as a standard under the same conditions.
4. Measurement of nitrite scavenging ability (NSA)
NSA was measured by applying the previous method of Panda et al.(2009) analyzed using Griess reagent. The 40 μL of each extract sample was mixed with 20 μL of 1 mM nitrite sodium. Then, the mixture was added to 140 μL of 0.2 M citrate buffer (pH 1.2, 4.2, or 6.0). The final volume of each sample wad adjusted to 200 μL. The mixture was incubated at 37°C, for 1 hr, and then 1000 μL of 2% acetic acid and 80 μL of Griess reagent were added. The incubated samples were mixed vigorously using a vortexer, and then the mixture placed for 15 min at room temperature. The NSA was measured in absorbance at 520 nm, and was determined according to the following formula:
NSA (%) = ((1-A-C)/B) × 100
A: the absorbance of the mixture during the reaction with 1 mM NaNO2 after 1 hr
B: the absorbance of a mixture of distilled water and 1 mM NaNO2 after 1 hr
C: the absorbance of the sample
5. Evaluation of in vitro anti-inflammatory activity
RAW 264.7 is a mouse monocyte-macrophage cell line established from the ascites of a tumor induced in a male mouse. RAW 264.7 cells were purchased from the Korean Cell Line Bank (KCLB) and were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and 1% antibiotic–antimycotic. The cells were maintained in a 5% CO2 incubator at 37°C.
The cytotoxicity of PG extract against RAW 264.7 cells was performed by MTT conversion assay. The RAW 264.7 cells were plated at a density of 1 × 104 cells/well in 96-well plates for 24 hr, followed by the addition of various concentrations (50, 100, 200, 400, and 800 μg·mL-1) of the PG extract. After 16 hr of incubation, 50 μg·mL-1 MTT solution (5 mg· mL-1) was added to each well and allowed to stand for 4 hr at 37°C. The medium was carefully removed to protect the formazan produced by the reduction of MTT and the formazan crystals were dissolved using 300 μg·mL-1 DMSO. Absorbance was measured at 540 nm using a UV-visible microplate reader (model 550; Bio-Rad, Hercules, CA, USA). Cell viability was calculated as sample OD/blank OD × 100 (%).
The RAW 264.7 cells were cultured in a 96-well plate and incubated for 18 hr at 37°C in 5% CO2. The cells were then incubated for 18 hr after treatment with 0.5 μg·mL-1 LPS (Sigma, St. Louis, MO, USA). The NO content was determined by the Griess reagent system. RAW 264.7 cells were seeded at a density of 5 × 104 cells/well in 6-well plates for 24 hr, pre-treated with 0.5 μg·mL-1 LPS, and added to the extract at various concentrations (50, 100, and 200 μg·mL-1). After the cells were incubated for 16 hr, NO levels were determined by the Griess reagent system using the supernatant. Absorbance was determined at 540 nm using a UV-visible microplate reader (Bio-Rad model 550, Hercules, CA, USA).
Murine RAW 264.7 peritoneal macrophage cells were cultured at a density of 1 × 104 cells/well in 6-well plates for 24 hr. They were then washed with phosphate-buffered saline (PBS) and treated with various concentrations (50, 100, and 200 μg·mL-1) of the GEB extract with 0.5 μg·mL-1 LPS for 24 hr. Supernatants were collected, and levels of IL-1β, IL-6, and TNF-α released into the culture supernatants were determined using commercially available cytokine ELISA kits (all from Bender R&D Systems, Inc.), according to the manufacturer’s instructions.
6. Data analysis
For all experiments, three to five independent replicates were evaluated. The statistical analyses were performed using Statistical Analysis System (version 9.1; SAS Institute Inc., Cary, NC, USA). The one-way ANOVA procedure followed by Duncan’s Multiple Range Tests were used to detect significant differences (p<0.05) in mean values among groups.
III. RESULTS AND DISCUSSION
1. DPPH radical scavenging activity
The comparative results of the DPPH free radical scavenging rate of PG adventitious root extract in different flower color and flower shape are shown in Table 1. DPPH is a stable nitrogen-centered free radical the color of which changes from violet to yellow upon reduction by either the process of hydrogen-or electron-donation. Substances which are able to perform this reaction can be considered as antioxidants and therefore radical scavengers (Dehpour et al. 2009). The PG extract of double petal purple color had the highest DPPH activity of 41.76% and the blue color PG extract showed the lowest DPPH activity of 34.57% at a concentration of 20 mg mL-1. All extracts showed a tendency to increase DPPH activity in a dose-dependent manner. The antioxidant activity of natural substances is determined based on the electron donor capacity of DPPH, which inhibits oxidation by donating electrons in free radicals, causing lipid peroxidation (Boo et al. 2012). Free radicals contribute to biological damage, and DPPH activity is an indicator of the free radical-scavenging activity of natural antioxidants (Kim et al. 2017). Cells are oxidized and damaged by the free radical, depending on the growth of cells. It has been reported that saponin components of PG have the antioxidant capacity to inhibit the oxidation by donating electrons to the free radical due to strong reduction (Kim et al. 2010; Ryu et al. 2012). The effective source of PG could be employed in all medicinal preparation to combat myriad diseases associated with oxidative stress. This results showed that PG could be employed in a medicinal preparation to combat diseases associated with oxidative stress.
2. ABTS radical scavenging activity
The formation of the ABTS radical cation takes place almost instantaneously after additing potassium persulfate to an ABTS solution. ABTS assays were performed in order to evaluate the radical scavenging activities of PG adventitious root extracts according to the PG flower color and flower shape. The results of the ABTS radical scavenging activity were similar to those for DPPH radical scavenging activity. That is, the PG extract of double petal purple color had the highest ABTS activity of 46.38% and the blue color PG extract showed the lowest ABTS activity of 33.33% (Table 2). All the tested samples at 20 mg mL-1 concentration exhibited effective radical cation scavenging activity. The ABTS radical scavenging activity was progressively increased in a dose-dependent manner. Therefore, the ABTS radical scavenging activity of adventitious root extract of PG indicates its ability to scavenge free radicals, thereby preventing oxidation via a chain-breaking reaction. Radical scavenging activities are very important due to the deleterious role of free radicals in foods and in biological systems. However, the antioxidant activity was low in this adventitious root extract compared to the results of the general PG root extract in our previous study. The electron-donating ability is determined by the one electron oxidation potential of the parent antioxidants, expressed by definition as the reduction potential of the corresponding phenoxyl radicals (Chan et al. 1998). In the present study, the results were supposed that PG adventitious root extracts were somewhat effective in antioxidant properties.
3. Nitrite scavenging activity (NSA)
The results of the determination of NSA of PG adventitious root extract according to the PG flower color and flower shape are summarized in the Table 3. It was examined over a range of acidic conditions (pH 1.2, 4.2 and 6.0). The NSA effect was the highest at pH 1.2 in all samples tested. However, there was no distinct detection of nitrite scavenging effects of the pH range 6.0. The highest scavenging ability (54.19%) was detected in extract of double petal purple color at a pH of 1.2, and the blue color root extract was the lowest. These results were also similar to the DPPH and ABTS results, and were considered to be correlated. The fact that the NSA was high at pH 1.2 suggests that nitrosamine production can be inhibited in vivo (Choi et al. 2008). These results were consistent with other findings that had the highest the nitrite scavenging at pH of 1.2 in fermented pine extract (Hong et al. 2004) and extracts from different parts of citron (Shin et al. 2005). These results suggested that the PG adventitious root extract have potent NSA and are potentially useful antioxidants in processed foods, and might be a source of food and natural antioxidants.
4. Cytotoxicity on RAW 264.7 cell
The cytotoxic effect of PG extracts on RAW 264.7 cell viability was assessed by the MTT assay. The cytotoxicity of the PG adventitious root extract according to the PG flower color and flower shape are summarized in the Table 4. When cells were treated for 2 days with various concentrations (50, 100, 200, 400 and 800 μg mL-1) of extracts, the rate of cell survival progressively decreased in a dose-dependent manner. As a result of treatment with PG adventitious root extract at various concentrations, the survival rate of RAW 264.7 cell was more than 90% at a concentration of 200 μg mL-1 or less, and it was confirmed that the macrophage cell showed little toxicity. Therefore, the anti-inflammatory assay was performed based on the concentration of less than 200 with little toxicity.
5. NO production
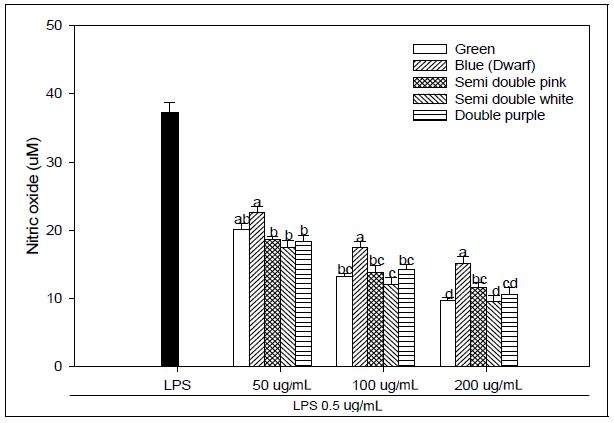
NO production from activated macrophages is essential for the control of a variety of microbial infections (Bogdan et al. 2000). Abnormalities in vascular NO production and transport result in endothelial dysfunction with various cardiovascular pathologies like hypertension, atherosclerosis and angiogenesis-associated disorders (Chen et al. 2008). NO production of the LPS alone was significantly higher than LPS-treated with PG extracts at 24 hr after pre-treatment at 50, 100, and 200 μg mL-1 with PG adventitious root extracts (Fig. 1). The dose-response trends followed quadratic regressions in all PG samples. NO production tended to be more decrease in the samples of green flower and white flower of semidouble petal than the other samples at all concentrations treated with PG extract., which significantly reduced NO production compared to LPS treatment alone (35.99 μM). In macrophages, NO is produced by the conversion of arginine and oxygen to citrulline and NO via inducible NO synthase (iNOS). iNOS levels in activated macrophages are regulated by complex transcriptional and posttranscriptional mechanisms (Taylor and Geller 2000). Macrophages are involved in the innate immune response via phagocytosis or the production of a variety of compounds, including cytokines and NO (Dempsey et al. 2003). NO is a reactive free radical that can produce the toxic compound peroxynitrite, high NO concentrations exert deleterious effects on lipids, DNA and proteins, similar to that observed with oxygen-derived species such as hydroxyl radicals. Although the mechanisms by which NO regulates the angiogenic process are not fully understood, NO has emerged as an important modulator of physiologic and pathologic angiogenesis and inflammation (Choi et al. 2009). Arctigenin blocked LPS-induced various responses of macrophage including the NO overproduction and the release of pro-inflammatory cytokines TNF-α and IL-6 (Zhao et al. 2009). These results suggest that PG adventitious root extracts have immunosuppressive potential; furthermore, these effects differ depending on the flower color and flower shape.
6. Cytokine production
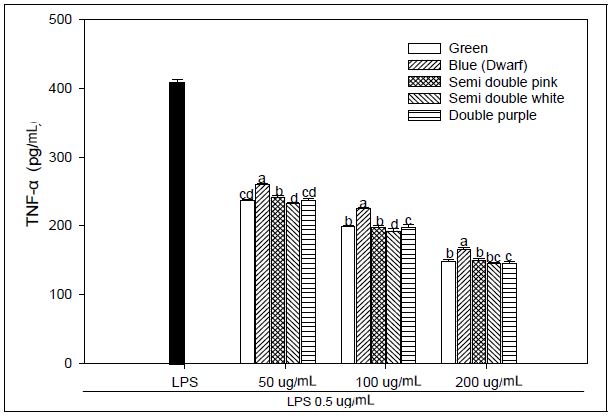
We explored that the PG extracts have an anti-inflammatory effect and operated LPS-stimulated macrophages as a method for in vitro assays. Macrophages were treated with LPS, and the concentrations of IL-1β, IL-6, and TNF-α secreted from the supernatant were evaluated. We used LPS-stimulated macrophages as a model for testing adventitious root extracts of PG for anti-inflammatory activity. Macrophages were treated with LPS, and the concentrations of secreted IL-1β, IL-6 and TNF-a in the supernatant were determined. We investigated the effect of PG adventitious root extracts on the expression of various pro-inflammatory and inflammatory cytokines induced by LPS in RAW 264.7 cells. As a result, IL-6 production at 200 μg mL-1 concentration of PG adventitious root extract showed the LPS 193.61 pg mL-1, green flower 92.92 pg mL-1, blue flower 106.90 pg mL-1, pink flower of semidouble petal 97.10 pg mL-1, white flower of semidouble petal 86.03 pg mL-1 and purple flower of double petal 96.57 pg mL-1, and it was significantly inhibited in a concentration-dependent manner (Fig. 2). IL-1β production at 200 μg mL-1 concentration of PG adventitious root extract showed the LPS 341.19 pg mL-1, green flower 146.08 pg mL-1, blue flower 185.35 pg mL-1, pink flower of semidouble petal 164.62 pg mL-1, white flower of semidouble petal 148.48 pg mL-1 and purple flower of double petal 157.54 pg mL-1, and it was also inhibited in a concentration-dependent manner (Fig. 3). TNF-α production at 200 μg mL-1 concentration of PG adventitious root extract showed the LPS 408.72 pg mL-1, green flower 148.35 pg mL-1, blue flower 166.22 pg mL-1, pink flower of semidouble petal 149.66 pg mL-1, white flower of semidouble petal 145.07 pg mL-1 and purple flower of double petal 144.86 pg mL-1, and it was also inhibited in a concentration-dependent manner (Fig. 4). In particular, these results showed that the anti-inflammatory activity was different depending on the PG flower color and flower shape, and the efficacy was relatively high in the double petal purple color and semidouble petal white color. Upon stimulation of the LPS, monocytes produce proinflammatory cytokines, such as TNF-α, IL-6, and IL-1β. Persistent inflammatory responses can damage host tissues (Michalaki et al. 2004). The inflammatory mediators TNF-α, IL-6 and IL-1β are known to regulate the inflammatory response in both in vivo and in vitro. These cytokines are known to interact with each other and are reported to be induced by inflammatory stimuli such as LPS (Feldmann et al. 1996). Inflammation not only plays a role in the inflammatory diseases, but also in the progression of cancer. Several inflammatory stages have been shown to predispose patients to cancer, such as inflammatory bowel disease, predisposing patients to colorectal cancer, H. pylori-induced gastritis to gastric cancer, or prostatitis to prostate cancer (Balkwill et al. 2005). The present study demonstrated an enhanced anti-inflammatory response in an LPS-stimulated macrophage example following cure with PG adventitious root extracts through reductions in IL-6, TNF-α, and IL-1β production or a reduction in the expression of NO. These results suggest that PG adventitious root extracts have potential anti-inflammatory activity, however, further in-depth studies are needed to promote their practical application.
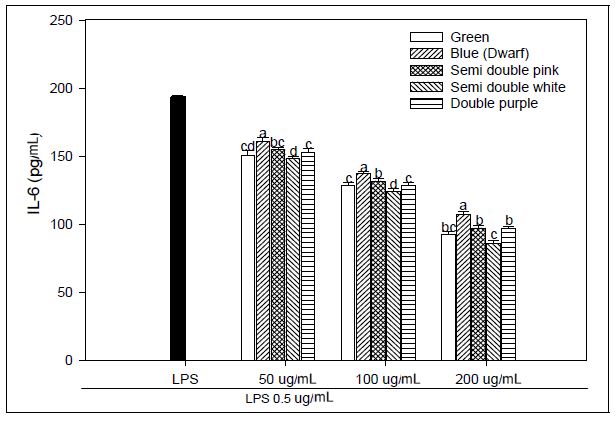
Effect of Platycodon grandiflorum adventitious root extracts on LPS-induced IL-6 production in RAW 264.7 cells. Within an extract concentration, the means followed by the same letter are not significantly different at p<0.05. Bars represent SE.
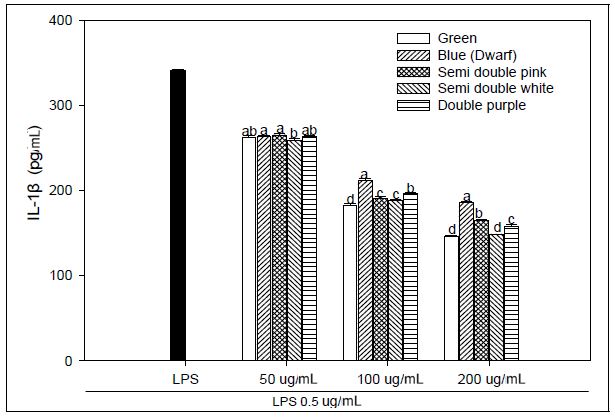
Effect of Platycodon grandiflorum adventitious root extracts on LPS-induced IL-1β production in RAW 264.7 cells. Within an extract concentration,the means followed by the same letter are not significantly different at p<0.05. Bars represent SE.
IV. SUMMARY AND CONCLUSIONS
The results obtained in the present study demonstrated the responses of the PG adventitious root extracts related to anti-inflammatory and antioxidant activity according to PG flower color and flower shape. Three free radicals were used to assess the potential free radical-scavenging activities of PG adventitious root extracts, namely DPPH radical, ABTS radical, and nitrite radicals. The results prevailed that the anti-inflammatory responses from TNF-α, IL-6, and IL-1β were influenced by the dose-dependent manner and most of the studied extracts have potential activity. Taken Together, this study indicates that bioactive molecule present in PG adventitious root could be helpful for the development of new drugs and / or as a source of basic medicine in the treatment of some diseases.
Acknowledgments
This study was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through Export Promotion Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (grant number 116121-03-2-HD030).
References
-
Balkwill F, Charles KA, Mantovani A(2005) Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7(3), 211-217.
[https://doi.org/10.1016/j.ccr.2005.02.013]

-
Bogdan C, Rollinghoff M, Diefenbach A(2000) Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol 2, 64-76.
[https://doi.org/10.1016/S0952-7915(99)00052-7]

-
Boo HO, Shin JH, Shin JS, Choung ES, Bang MA, Choi KM, Song WS(2012) Assessment on Antioxidant Potential and Enzyme Activity of Some Economic Resource Plants. Korean J Plant Res 25(3), 349-356.
[https://doi.org/10.7732/kjpr.2012.25.3.349]

-
Brand-Williams W, Cuvelier ME, Berset C(1995) Use of a free radical method to evaluate antioxidant activity. Food Sci Tech 28, 25-30.
[https://doi.org/10.1016/S0023-6438(95)80008-5]

-
Chan MM, Huang HI, Fenton MR, Fong D(1998) In vivo inhibition of nitric oxide synthase gene expression by curcumin, a cancer preventive natural product with anti-inflammatory properties. Biochem Pharmacol 55, 1955-1962.
[https://doi.org/10.7732/kjpr.2012.25.3.349]

-
Chen K, Pittman RN, Popel AS(2008) Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal 10, 1185-1198.
[https://doi.org/10.1089/ars.2007.1959]

-
Choi DB, Cho KA, Na MS, Choi HS, Kim YO, Lim DH, Cho SJ, Cho H(2008) Effect of bamboo oil on antioxidative activity and nitrite scavenging activity. J Ind Eng Chem 14, 765-770.
[https://doi.org/10.1016/j.jiec.2008.06.005]

-
Choi EJ, Debnath T, Tang Y, Ryu YB, Moon SH, Kim EK (2016) Topical application of Moringa oleifera leaf extract ameliorates experimentally induced atopic dermatitis by the regulation of Th1/Th2/Th17 balance. Biomed & Pharmaco 84, 870-877
[https://doi.org/10.1016/j.biopha.2016.09.085]

-
Choi YS, Choi HJ, Min JK, Pyun BJ, Maeng YS, Park HR, Kim PJ, Kim YM, Kwon YG(2009) Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood 114(14), 3117-3126.
[https://doi.org/10.1182/blood-2009-02-203372]

-
Cook NC, Samman S(1996) Flavonoids―Chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem 7(2), 66-76.
[https://doi.org/10.1016/S0955-2863(95)00168-9]

-
Dehpour AA, Ebrahimzadeh MA, Nabavi SF, Nabavi SM(2009) Antioxidant activity of methanol extract of Ferula assafoetida and its essential oil composition. Grasas Aceites 60(4), 405-412.
[https://doi.org/10.3989/gya.010109]

-
Dempsey PW, Vaidya SA, Cheng G(2003) The art of war: Innate and adaptive immune response. Cell Mol Life Sci 60, 2604-2621.
[https://doi.org/10.1007/s00018-003-3180-y]

-
Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, Ju YH(2014) Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatic. J Food Drug Anal 22, 296-302.
[https://doi.org/10.1016/j.jfda.2013.11.001]

-
Feldmann M, Brennan FM, Maini RN(1996) Role of cytokines in rheumatoid arthritis. Annual Rev Immunol 14, 397-440.
[https://doi.org/10.1146/annurev.immunol.14.1.397]

-
Gülçin I(2010) Antioxidant properities of resveratrol: a structure-activity insight. Innov Food Sci Emerg Technol 11, 210-218.
[https://doi.org/10.1016/j.ifset.2009.07.002]

- Hong TG, Lee YR, Yim MH, Hyun CN(2004) Physiological functionality and nitrite scavenging ability of fermentation extracts from pine needles. Korean J Food Preserv 11, 94-99
- Kim CH, Jung BY, Jung SK, Lee CH, Lee HS, Kim BH, Kim SK(2010) Evaluation of antioxidant activity of Platycodon grandiflorum. J Environ Toxicol 25(1), 85-94
-
Kim ID, Dhungana SK, Kim HR Shin, DH(2017) Quality characteristics and antioxidant potential of seeds of native Korean persimmon genotypes. Korean J Plant Res 30(6), 670-678.
[https://doi.org/10.7732/kjpr.2017.30.6.670]

-
Michalaki V, Syrigos K, Charles P, Waxman J(2004) Serum levels of IL-6 and TNF-a correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer 90, 2312-2316
[https://doi.org/10.1038/sj.bjc.6601814]

- Panda BN, Raj AB, Shrivastava NR, Prathani AR(2009) The evaluation of nitric oxide scavenging activity of Acalypha indica Linn Root. Asian J Res Chem 2(2), 148-150
-
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Evans CR(1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio Med 26(9-10), 1231-1237.
[https://doi.org/10.1016/S0891-5849(98)00315-3]

-
Ryu CS, Kim CH, Lee SY, Lee KS, Choung KJ, Song GY, Kim BH, Ryu SY, Lee HS, Kim SK(2012) Evaluation of the total oxidant scavenging capacity of saponins isolated from Platycodon grandiflorum. Food Chem 132(1), 333-337.
[https://doi.org/10.1016/j.foodchem.2011.10.086]

-
Shin JH, Lee JY, Ju JC, Lee SJ, Cho HS, Sung NJ(2005) Chemical properties and nitrite scavenging ability of citron (Citrus junos). J Korean Soc Food Sci Nutr 34, 496-502.
[https://doi.org/10.3746/jkfn.2005.34.4.496]

-
Taylor BS, Geller DA(2000) Molecular regulation of the human inducible nitric oxide synthase (iNOS) gene. Shock 13, 413-424.
[https://doi.org/10.1097/00024382-200006000-00001]

-
Gao W, Guo Y, Yang H(2017) Platycodin D protects against cigarette smoke-induced lung Inflammation in mice. Int Immunopharmacol 47, 53-58.
[https://doi.org/10.1016/j.intimp.2017.03.009]

-
Zhao F, Wang L, Liu K(2009) In vitro anti-inflammatory effects of arctigenin, a lignan from Arctium lappa L., through inhibition on iNOS pathway. J Ethnopharacol 122, 457-462.
[https://doi.org/10.1016/j.jep.2009.01.038]