Comparison of Total Polyphenol, Total Flavonoid Content and Antioxidant Activity of Codonopsis lanceolata Extracts Stored at Different Temperatures and for Different Durations
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This study was conducted to investigate changes in phenolic and flavonoid content and antioxidant activity according to storage temperature and storage period from root extract of Codonopsis lanceolata (CL). Total polyphenol and flavonoid content decreased with increasing temperature, and the content was relatively high at temperatures of 15°C or less. Under different storage conditions, the total polyphenol and flavonoid content tended to decrease as the storage period became longer. Measurement of the DPPH radical scavenging activity confirmed that components for enhancing antioxidants were contained in the roots of CL, and it was assumed that there was not a large change in the scavenging activity when stored for less than 60 days or at temperatures below 25°C. Shorter storage period and lower storage temperature was associated with relatively high ABTS radical scavenging activity. The nitrite scavenging effect was highest at pH 1.2 in all samples tested; however, there was no distinct detection of nitrite scavenging effects at pH 6.0. In addition, phenolic compounds appear to be responsible for the antioxidant activity of CL extracts. Therefore, the results of the current study suggest that the CL root may assist in potential biological activities and can be used as a source of human health products.
Keywords:
Codonopsis lanceolata, ABTS, DPPH, flavonoid, nitrite scavenging activity, polyphenolI. Introduction
As an herb, Codonopsis lanceolata(CL) is widely used in food preparation, but its medicinal application has not been explored yet in South Korea(Wang et al. 2011). The roots of CL have been used as a tonic crude drug and an edible plant in Korea, and it is well known to affect various pharmacological effects for human health and its consumption is increasing. Recently, plant and plant-derived products are treated a part of the healthcare system by applying the bioactive phytochemicals. Medicinal plants are belived to be a potential source for the research of new biologically active compounds. A part from the medicinal effects of traditional herbs, exploratoty researches have been executed and a vast variety of new biological activities from traditional medicinal plants have recently been reported, including anticancer activity(Pittella et al. 2009). Phenolic compounds include phenolic acids (hydroxybenzoic and hydroxycinnamic acids), polyphenols(hydrolyzable and condensed tannins), and flavonoids. These compounds protect plants, fruits, and vegetables from oxidative damage and have been used as antioxidants by humans. Finding new and safe antioxidants from natural sources is of great interest for applications in natural antioxidants, functional foods, and neutraceuticals. Phytochemical screening is one of the methods that have been used to explore antioxidant compounds in plants(Do et al. 2014). Phenolics possess a wide spectrum of biochemical activities such as antioxidant, antimutagenic, anticarcinogenic, as well as ability to modify the gene expression (Tapiero et al. 2002; Nakamura et al. 2003). Numerous epidemiological studies confirm significant relationship between the high dietary intake of flavonoids and the reduction of cardiovascular and carcinogenic risk(Cook & Samman 1996). Antioxidant compounds can scavenge free radicals and increase shelf life by retarding the process of lipid peroxidation, which is one of the major reasons for deterioration of food and pharmaceutical products during processing and storage(Gülçin 2010). Antioxidants can protect the human body from free radicals and reactive oxygen species(ROS) effects. They retard the progress of many chronic diseases as well as lipid peroxidation. Hence, a need for identifying alternative natural and safe sources of food antioxidants has been created, and the search for natural antioxidants, especially of plant origin, has notably increased in recent years(Gülçin et al. 2004). Antioxidant compounds in food play an important role as a health protecting factor. Scientific evidence suggests that antioxidants reduce the risk for chronic diseases, including cancer and heart disease. Antioxidants from aromatic, spicy, medicinal, and other plants were studied to develop natural antioxidant formulations for food, cosmetic, and other applications(Miliauskas et al. 2004). Most of the antioxidant compounds in a typical diet are derived from plant sources and belong to various classes of compounds with a wide variety of physical and chemical properties. CL is a medicinal plant having effective pharmacological functionality, but has a disadvantage that it is poorly stored. Therefore, it is very important to investigate the storage temperature and storage period in which the physiological functionality of CL is maintained. The objection of this study was to investigate the change of phenolic content according to storage temperature and storage period from root extract of CL, and investigate the antioxidant activity of the extracts by in vitro methods.
II. MATERIALS AND METHODS
1. Plant material and extract preparation
Codonopsis lanceolata grown in Jeju region was purchased from a farm. The roots of CL at different temperatures(5°C, 15°C, 25°C, 35°C, and 45°C) using thermo-chamber and storage periods(0, 15, 30, 45, 60, 75, and 90 d) were freeze dried and ground to a fine powder. The powder was stored at -20°C until further analyses. The freeze dried powder was mixed with 30% ethanol and the filtrate was collected thrice with constant stirring of the mixture at every 24 h interval for 72 h. The filtrate was then concentrated under reduced pressure at 45°C using a vacuum rotary evaporator(IKA® RV 10 Basic Digital, IKA Co., Germany). The concentrated extract was stored at -20°C until further analysis
2. Total polyphenol determination
Total phenols were determined by the modified method the Folin-Ciocalteu assay(Singleton & Rossi 1965). Freeze-dried samples were extracted with methanol, the extract was concentrated under reduced pressure, and freeze-dried in powder. 1 mg freeze-dried powder dissolved in 95% methanol, and 500 μL of Folin-Ciocalteu reagent were added to a 25 mL volumetric flask and were mixed for 5 minute at 30°C in water bath. 500 μL saturated solution of 7.5% Na2CO3 was added to the mixture, and then was incubated for 1 hour at room temperature, and the absorbance was read at 725 nm using a spectrophotometer(Biochrom Co., England). Total phenolic of the sample was expressed as mg chlorogenic acid equivalent in 1 g dry weight of sample extract.
3. Total flavonoid determination
Total flavonoid was measured using the modified method that previously described(Zhishen et al. 1999). Briefly, 1 mg freeze-dried samples dissolved in 95% methanol, and 1 mL of extract solution, 10 mL diethylene glycol and 0.1 mL 1N NaOH were added to a 25 mL volumetric flask. The mixture was incubated for 1 hour at 37°C in water bath. The absorbance was measured at 420 nm using a spectrophotometer(Biochrom Co., England). Total flavonoid of the samples was expressed as mg narincin equivalent in 1 g dry weight of sample extract.
4. Assay of DPPH radical scavenging rate
100 μL of various concentrations(2.5, 5, 10 and 20 mg/mL) of extracts in CL were added to 900 μL of 100% methanol containing 100 μM DPPH, and the reaction mixture was shaken for 5 min in the slight vortex. Leaving room temperature for 30 min under darkness, the absorbance of DPPH was determined by spectrophotometer at 517 nm. The DPPH radical scavenging activity was calculated according to the following equation: Scavenging effect on DPPH radical(%) = [(A-B)/A]x100, Where A is the absorbance at 517 nm without pigment compositions and B is the change in absorbance at 517 nm with pigment compositions incubation (Brand-Williams et al. 1995).
5. Assay of ABTS radical scavenging rate
The spectrophotomeric analysis of ABTS (2,2'-azinbis-(3-ethyl-benzothiazoline-6-sulfonicacid) radical cation(ABTS•+) scavenging activity of CL was determined according to the method described previously(Re et al. 1999). 7 mM ABTS solution with 2.45 mM potassium persulfate was mixed, and the mixture was incubated in the dark at room temperature for 15 h, and then was diluted to the absorbance 0.7 at 734 nm. Fifty μL of each sample prepared in different concentrations with 950 μL diluted solution was added, and was shaken for 10 seconds by vortex mixer, and then was reacted for 5 min at room temperature, and the absorbance was read at 734 nm using a spectrophotometer(Biochrom Co., England). The ABTS•+ scavenging activity showed as RAEAC (relative ascorbic acid equivalent antioxidant capacity), was calculated by the following equation:
ΔAaa: change of the absorbance after addition of ascorbic acid
Caa: concentration of ascorbic acid
ΔAs: change of the absorbance after addition of sample solution
Cs: concentration of sample
6. Assay of Nitrite scavenging rate
The nitrite scavenging activity(NSA) was determined according to a method using Griess reagent(Kato et al. 1987). First, 40 μL of each sample was mixed with 20 μL of 1 mM nitrite sodium. Then the mixture was added to 140 μL of 0.2 M citrate buffer(pH 1.2, 4.2, or 6.0). The final volume of each sample wad adjusted to 200 μL. After, the mixtures had been incubated for 1 h at 37˚C, and added to 1000 μL of 2% acetic acid and 80 μL of Griess reagent(1% sulfanilic acid and 1% naphthylamine in a methanol solution containing 30% acetic acid). After vigorous mixing with a vortex, the mixture was placed at room temperature for 15 min, and absorbance was measured at 520 nm. The nitrite scavenging activity was determined based on the following formula:
Where A is the absorbance of the mixture sample during a reation with 1 mM NaNO2 after a 1 h reaction, B is the absorbance of a mixture of distilled water and 1 mM NaNO2 after a 1 h reaction and C is the absorbance of the sample.
7. Data analysis
The statistical analysis was performed using the procedures of the Statistical Analysis System(SAS version 9.1). The ANOVA procedure followed by Duncan test was used to determine the significant difference(p<0.05) between treatment means.
III. RESULTS AND DISCUSSION
1. Total polyphenol and flavonoid contents
Plant phenolics constitute one of the major groups of compounds acting as primary antioxidants or free radical terminators. Polyphenols are widely distributed in plants and phenolic antioxidants have been found to act as free radical scavengers as well as metal chelators(Shahidi & Wanasundara 1992; Sanchez-Moreno et al. 1999). It has been recognized that the compounds such as polyphenol, flavonoid show antioxidant activity and their effects on human nutrition and health are considerable. The results for total polyphenol and total flavonoid content according to storage temperature and storage period in the CL root extracts are presented in table 1 and 2. Total polyphenol and flavonoid content by different storage temperature conditions decreased with increasing temperature, and the content was relatively high at low temperature of 15°C or less. In different storage period conditions, the total polyphenol and flavonoid content tended to decrease as the storage period became longer. These results were considerably consistent with the finding of antioxidant activity. A number of studies have focused on the biological activities of phenolic compounds, which are potential antioxidants and free radical scavengers(Marja et al. 1999; Sugihara et al. 1999). Zhou & Yu(2006) also reported that total phenolic content of the tested vegetable extracts was correlated with the DPPH radical scavenging activity, suggesting that total phenolics can play a major role in the antioxidant activity of plant materials. Some medicinal plants traditionally used for management of diseases were selected and their phenol and flavonoid content and iron chelating activities were evaluated(Ebrahimzadeh et al. 2008). Rivera-Pastrana et al.(2010) reported that phenolic and carotenoid profiles of papaya fruit mesocarp and exocarp tissues were identical at chilling(1°C) and control(25°C) temperatures, and differences were found among individual compounds in response to storage temperature and duration. Other studies also showed that the reduction in total polyphenol content could be explained by the convertion between free and bound phenolic substances, which can also be affected by storage temperature and storage period(Ferrante & Maggiore 2007; Serea et al. 2014). These results are consistent with the results of this study. The formulation of preventive and healthy nutrition requires information about phenolic and flavonoid composition in plant foods. In order to confirm the optimal storage condition of the CL root, current study was focused on determination of total phenolic and total flavonoid content in CL extract.

Total polyphenol and flavonoid contents of root extracts from Codonopsis lanceolata according to different storage periods
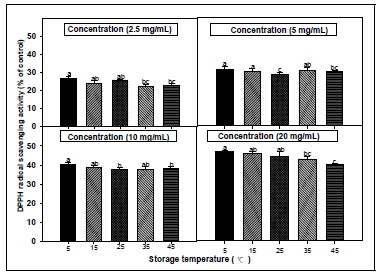
2. DPPH radical scavenging activity
DPPH assay has been widely used to evaluate the free radical scavenging effectiveness of various antioxidant substances(MacDonald-Wicks et al. 2006). DPPH is a stable nitrogen-centered free radical the color of which changes from violet to yellow upon reduction by either the process of hydrogen- or electron- donation. Substances which are able to perform this reaction can be considered as antioxidants and therefore radical scavengers (Dehpour et al. 2009). Fig. 1 and 2 shows the DPPH free radical scavenging activities of the CL extracts at a different stiorage temperature and storage period conditions. In different storage period condition, the DPPH radical scavenging activity of CL root extract decreased a concentrationdependent manner as the storage period becomes loger. The DPPH radical scavenging activity of root extract from CL in different temperature condition was increased when the storage temperature was low. As a result of measuring the DPPH radical scavenging activity, it was confirmed that the component for enhancing antioxidant was contained in the root of CL, and it was presumed that there was not a large change in the scavenging activity when stored for below 60 days or at temperature below 25°C. The investigation of the antioxidant activity of natural substances is based on the measuring of the electron donor capacity of DPPH with the ability to inhibit the oxidation by donating electrons in free radicals causing this lipid peroxidation(Boo et al. 2012), that is, free radical are known to be a major factor in biological damages, and DPPH has been used to evaluate the free radical-scavenging activity of natural antioxidants (Zhu et al. 2001; Kim et al. 2017). Active oxygen caused by in vivo metabolism removed by the body’s antioxidant system, but excessive free radicals induced stress, causing the lipid peroxidation by combining with unsaturated fatty acids in the cell membrane, and brought intracellular structural and functional damage. The effective source of CL could be employed in all medicinal preparation to combat myriad diseases associated with oxidative stress. Phenolics were the main antioxidant components, and their total contents were directly proportional to their antioxidant activity(Liu et al. 2009). Usually, higher phenolic and flavonoid content lead to better DPPH scavenging activity.
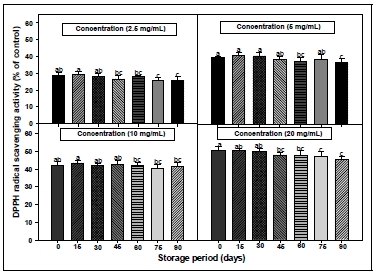
DPPH radical scavenging activities of root extracts from Codonopsis lanceolata according to different storage period conditions. The bars represent the standard error. Means followed by the same letter are not significantly different at p<0.05.
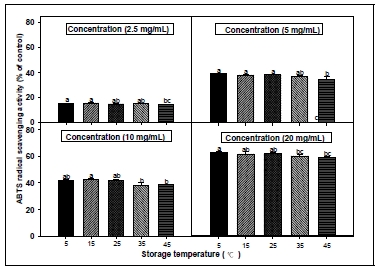
3. ABTS radical scavenging activity
The ABTS and DPPH systems have both been commonly used to measure the total antioxidative status of various biological specimens because of their good reproducibility and easy quality control(Brand-Williams et al. 1995; Re et al. 1999). The formation of the ABTS radical cation takes place almost instantaneously after additing potassium persulfate to an ABTS solution. In order to evaluate the radical scavenging activities of extracts according to the storage period and storage temperature from CL, ABTS assays were performed. The results of the ABTS radical scavenging activity were shown in Fig. 3 and 4. All the tested samples exhibited effective radical cation scavenging activity. When the experimental samples from CL root were treated with various concentrations(2.5, 5, 10 and 20 mg/mL) of extracts, the ABTS radical scavenging activity was progressively increased in a dose- dependent manner. The shorter the storage period and the lower the storage temperature, the ABTS radical scavenging activity was relatively high. In particular, the ABTS radical scavenging activity decreased with the lengthening of the storage period. This suggests that storage temperature and storage period are important to maintain the physiological functionality of CL such as antioxidant. Phenolic antioxidants usually scavenge free radicals by an electron-transfer mechanism. The electron- donating ability is determined by the oneelectron oxidation potential of the parent antioxidants, expressed by definition as the reduction potential of the corresponding phenoxyl radicals(Chan et al. 1998). The ABTS radicals are scavenged by antioxidants via the mechanism of electron-/ hydrogen-donation. In the present study, interesting results were noted for the scavenger properties against ABTS radicals. It was observed that the tested CL extracts showed higher ability to scavenge ABTS radicals than DPPH radicals.
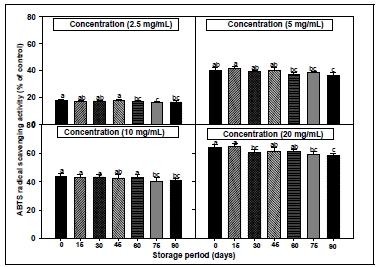
ABTS radical scavenging activities of root extracts from Codonopsis lanceolata according to different storage period conditions. The bars represent the standard error. Means followed by the same letter are not significantly different at p<0.05.
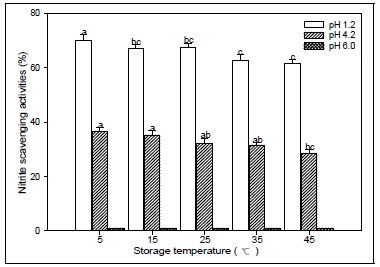
4. Nitrite scavenging activity
The results of the determination of nitrite scavenging activity of CL extract according to storage period and storage temperature condition are summarized in the Fig. 5 and 6. It was examined over a range of acidic conditions(pH 1.2, 4.2 and 6.0). The nitrite scavenging effect was the highest at pH 1.2 in all samples tested. However, there was no distinct detection of nitrite scavenging effects of the pH range 6.0. Also, These results prevailed that the nitrite scavenging activity of CL was influenced by the storage conditions, and the radical effect decreased under long term storage and high temperature conditions. The fact that the nitrite scavenging activity was high at pH 1.2 suggests that nitrosamine production can be inhibited in vivo(Choi et al. 2008). These results were consistent with other findings that had the highest the nitrite scavenging at pH of 1.2 in fermented pine extract(Hong et al. 2004) and extracts from different parts of citron(Shin et al. 2005). Nitrite reacts with second and third grade amines to form nitrosamine in protein-rich foods, medicines, and residual pesticides. It is also present in large quantities in meat and both leafy and root vegetables. Nitrosamine is converted to diazoalkane (alkane nucleic acid), proteins, and intracellular components, which can increase the risk for cancer(Choi et al. 2008). Nitric oxide(NO) is basically generated from amino acid larginine by vascular endothelial cells, phagocytes and certain cells of the brain. The toxicity of nitric oxide becomes adverse when it reacts with superoxide radical, forming a highly reactive peroxyritrite amino (ONOO-)(Boora et al. 2014). Nitrite is toxic and the consumption of excess nitrites over time results in the oxidization of hemoglobin, which can lead to methemoglobinemia(Jeon et al. 2002). It has been reported that phenolic compounds have a greater nitrite scavenging effect in environments with low pH(Noh et al. 2002). All results stated above suggested that the extract of CL root could be used as an antioxidant or nitrite scavenger in food industry and the utilization of the root extract could improve the whole economical value of CL. That is, the CL plant have potent nitrite scavenging activities and are potentially useful antioxidants in processed foods, and might be a source of food and natural antioxidants.
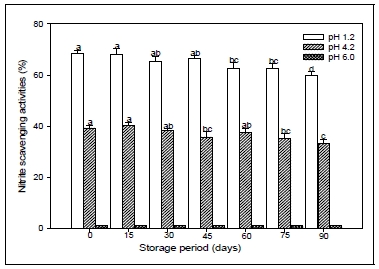
Nitrite scavenging activities of root extracts from Codonopsis lanceolata according to different storage period conditions. The bars represent the standard error. Means followed by the same letter are not significantly different at p<0.05.
IV. SUMMARY AND CONCLUSIONS
This study was conducted to investigate the change of phenolic, flavonoid content and antioxidant activity according to storage temperature and storage period from root extract of CL. Three free radicals were used to assess the potential free radical-scavenging activities of CL extracts, namely DPPH radical, ABTS radical, and nitrite radicals. In the present study, the extracts from CL were found to possess antioxidant activity. The antioxidant mechanisms of CL root extracts may be attributed to their free radical-scavenging ability. A positive relationship between antioxidant activities and total phenolic contents was also observed. In addition, phenolic compounds appear to be responsible for the antioxidant activity of CL extracts. The presented data for antioxidant activity and content of phenolic compounds of CL are a basis for assessment the optimum storage temperature and storage period, and will be useful for setting storage conditions. That is, it was thought that the storage condition after harvest of CL can be maintained in good quality by storing within 60 days at temperature below 25℃. Therefore, the results of current study suggested that the CL root may assist in the potential biological activities, and can be used as a source of human health products.
Acknowledgments
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry(IPET) through High Value-added Food Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs(MAFRA) (grant number 114036-04-4-SB010).
References
-
Boo, HO, Shin, JH, Shin, JS, Choung, ES, Bang, MA, Choi, KM, Song, WS, (2012), Assessment on antioxidant potential and enzyme activity of some economic resource plants, Korean J Plant Res, 25(3), p349-356.
[https://doi.org/10.7732/kjpr.2012.25.3.349]

-
Boora, F, Chirisa, E, Mukanganyama, S, (2014), Evaluation of nitrite radical scavenging properties of selected zimbabwean plant extracts and their phytoconstituents, J Food Process, 2014, p1-7.
[https://doi.org/10.1155/2014/918018]

-
Brand-Williams, W, Cuvelier, ME, Berset, C, (1995), Use of a free radical method to evaluate antioxidant activity, Food Sci Tech, 28, p25-30.
[https://doi.org/10.1016/s0023-6438(95)80008-5]

-
Chan, MM, Huang, HI, Fenton, MR, Fong, D, (1998), In vivo inhibition of nitric oxide synthase gene expression by curcumin, a cancer preventive natural product with anti-inflammatory properties, Biochem Pharmacol, 55, p1955-1962.
[https://doi.org/10.1016/s0006-2952(98)00114-2]

-
Choi, DB, Cho, KA, Na, MS, Choi, HS, Kim, YO, Lim, DH, Cho, SJ, Cho, H, (2008), Effect of bamboo oil on antioxidative activity and nitrite scavenging activity, J Ind Eng Chem, 14, p765-770.
[https://doi.org/10.1016/j.jiec.2008.06.005]

-
Cook, NC, Samman, S, (1996), Flavonoids―Chemistry, metabolism, cardioprotective effects, and dietary sources, J Nutr Biochem, 7(2), p66-76.
[https://doi.org/10.1016/0955-2863(95)00168-9]

- Dehpour, AA, Ebrahimzadeh, MA, Nabavi, SF, Nabavi, SM, (2009), Antioxidant activity of methanol extract of Ferula assafoetida and its essential oil composition, Grasas Aceites, 60(4), p405-412.
-
Do, QD, Angkawijaya, AE, Tran-Nguyen, PL, Huynh, LH, Soetaredjo, FE, Ismadji, S, Ju, YH, (2014), Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatic, J Food Drug Anal, 22, p296-302.
[https://doi.org/10.1016/j.jfda.2013.11.001]

- Ebrahimzadeh, MA, Pourmorad, F, Bekhradnia, AR, (2008), Iron chelating activity, phenol and flavonoid content of some medicinal plants from Iran, Afr J Biotechnol, 7(18), p3188-3192.
-
Ferrante, A, Maggiore, T, (2007), Chlorophyll a fluorescence measurements to evaluate storage time and temperature of Valeriana leafy vegetables, Postharvest Biol Technol, 45(1), p73-80.
[https://doi.org/10.1016/j.postharvbio.2007.02.003]

- Gülçin, I, (2010), Antioxidant properities of resveratrol: a structure-activity insight, Innov Food Sci Emerg Technol, 11, p210-218.
- Gülçin, I, Mshvildadze, V, Gepdiremen, A, Elias, R, (2004), Antioxidant activity of saponins isolated from ivy: a-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside F, Planta Medica, 70, p561-563.
- Hong, TG, Lee, YR, Yim, MH, Hyun, CN, (2004), Physiological functionality and nitrite scavenging ability of fermentation extracts from pine needles, Korean J Food Preserv, 11, p94-99.
- Jeon, TW, Jo, CH, Kim, KH, Byun, MW, (2002), Inhibitory effect on tyrosinase and xanthine oxidase, and nitrite scavenging activities of Schizandrae Fructus extract by gamma irradiation, Korean J Food Preserv, 9, p369-374.
-
Kato, H, Lee, IE, Van, Chuyen N, Kim, SB, Hayase, F, (1987), Inhibition of nitrosamine formation by nondialyzable melanoidins, Agr Biol Chem, 51, p1333-1338.
[https://doi.org/10.1080/00021369.1987.10868212]

- Kim, ID, Dhungana, SK, Kim, HR, Shin, DH, (2017), Quality characteristics and antioxidant potential of seeds of native Korean persimmon genotypes, Korean J Plant Res, 30(6), p670-678.
-
Liu, SC, Lin, JT, Wang, CK, (2009), Antioxidant properties of various solvent extracts from lychee (Litchi chinenesis sonn.) flowers, Food Chem, 114, p577-581.
[https://doi.org/10.1016/j.foodchem.2008.09.088]

-
MacDonald-Wicks, LK, Wood, LG, Garg, ML, (2006), Methodology for the determination of biological antioxidant capacity in vitro: a review, J Sci Food Agri, 86, p2046-2056.
[https://doi.org/10.1002/jsfa.2603]

- Marja, PK, Anu, IH, Heikki, JV, Jussi-Pekka, R, Kalevi, P, Tytti, SK, Marina, H, (1999), Antioxidant activity of plant extracts containing phenolic compounds, J Agri Food Chem, 47, p3954-3962.
-
Miliauskas, G, Venskutonis, PR, Van, Beek TA, (2004), Screening of radical scavenging activity of some medicinal and aromatic plant extracts, Food Chem, 85, p231-237.
[https://doi.org/10.1016/j.foodchem.2003.05.007]

- Nakamura, Y, Watanabe, S, Miyake, N, Konho, H, Osawa, T, (2002), Dihydrochalcones: evaluation as novel radical scavenging antioxidants, J Agric Food Chem, 51, p3309-3312.
- Noh, KS, Yang, MO, Cho, EJ, (2002), Nitrite scavenging effect of Umbelligeraeceae, Korean J Food Cook Sci, 18, p8-12.
-
Pittella, F, Dutra, RC, Junior, DD, Lopes, MT, Barbosa, NR, (2009), Antioxidant and cytotoxic activities of Centella asiatica (L) Urb, Int J Mol Sci, 10, p3713-3721.
[https://doi.org/10.3390/ijms10093713]

-
Re, R, Pellegrini, N, Proteggente, A, Pannala, A, Yang, M, Evans, CR, (1999), Antioxidant activity applying an improved ABTS radical cation decolorization assay, Free Radical Bio Med, 26(9-10), p1231-1237.
[https://doi.org/10.1016/s0891-5849(98)00315-3]

-
Rivera-Pastrana, DM, Yahiab, EM, Gonz´alez-Aguilara, GA, (2010), Phenolic and carotenoid profiles of papaya fruit (Carica papaya L.) and their contents under low temperature storage, J Sci Food Agric, 90, p2358-2365.
[https://doi.org/10.1002/jsfa.4092]

- Sanchez-Moreno, C, Larrauri, JA, Saura-Calixto, F, (1999), Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents, Food Res Int, 32, p407-412.
- Serea, C, Barna, O, Manley, M, Kidd, M, (2014), Effect of storage temperature on the ascorbic acid content, total phenolic content and antioxidant activity in lettuce (lactuca sativa L.), J Anim Plant Sci, 24(4), p1173-1177.
- Shahidi, F, Wanasundara, PKJPD, (1992), Phenolic antioxidants: criteria review, Food Sci Nutr, 32, p67-103.
- Shin, JH, Lee, JY, Ju, JC, Lee, SJ, Cho, HS, Sung, NJ, (2005), Chemical properties and nitrite scavenging ability of citron (Citrus junos), J Korean Soc Food Sci Nutr, 34, p496-502.
- Singleton, V, Rossi, JA, (1965), Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents, Am J Enology Vitic, 16, p144-158.
- Sugihara, N, Arakawa, T, Ohnishi, M, Furuno, K, (1999), Anti and pro-oxidative effects of flavonoids on metal induced lipid hydroperoxide-dependent lipid peroxidation in cultured hepatocytes located with "linolenic acid, Free Radic Biol Med, 27, p1313-1323.
-
Tapiero, H, Tew, KD, Ba, GN, Mathe, G, (2002), Polyphenols: do they play a role in the prevention of human pathologies?, Biomed Pharmacother, 56(4), p200-207.
[https://doi.org/10.1016/s0753-3322(02)00178-6]

-
Wang, L, Xu, ML, Hu, JH, Rasmussen, SK, Wang, MH, (2011), Codonopsis lanceolata extract induces G0/G1 arrest and apoptosis in human colon tumor HT-29 cells – Involvement of ROS generation and polyamine depletion, Food Chem Toxicol, 49, p149-154.
[https://doi.org/10.1016/j.fct.2010.10.010]

-
Zhishen, J, Mengcheng, T, Jianming, W, (1999), The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals, Food Chem, 64, p555-559.
[https://doi.org/10.1016/s0308-8146(98)00102-2]

-
Zhou, K, Yu, L, (2006), Total phenolic contents and antioxidant properties of commonly consumed vegetables grown in Colorado, LWT, 39, p1155-1162.
[https://doi.org/10.1016/j.lwt.2005.07.015]

-
Zhu, N, Wang, M, Wei, GJ, Lin, JK, Yang, CS, Ho, CT, (2001), Identification of reaction products of (-)-epigallocatechin, (-)-epigallocatechin gallate and pyrogallol with 2,2diphenyl-1- picrylhydrazyl radical, Food Chem, 73, p345-349.
[https://doi.org/10.1016/s0308-8146(00)00308-3]