Antioxidant Activities of Powdered and Ultra-fine Powdered Ulmus Davidiana var. Japonica
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The antioxidant activity was examined on ultra-fine powdered (UFP) Ulmus davidiana (U. davidiana) var. japonica. The average particle size of UFP or commercial powdered (CP) was 1-3 μm or 100 μm. The extraction was performed using either ethanol (EtOH) or hot-water. Contents of phenolic compound of CP and UFP U. davidiana extracts in EtOH was 40.38 and 65.61 mg/g, respectively. In DPPH, EtOH extract of UFP U. davidiana showed a significantly greater activity than hot-water extract at 40 and 200 μg/mL. At 200 μg/mL, the activity was over 90% in all groups. The reducing power of UFP U. davidiana var. japonica in EtOH extraction was 74.3%, which was significantly greater than in other samples (p<0.05). In addition, reducing powder was significantly higher in UFP-EtOH than in other samples at all concentrations except for 0.32 μg/mL. The above results suggest that EtOH extraction of U. davidiana showed slightly higher DPPH radical scavenging activity, and ultra-fine powder of U. davidiana extracts may show higher antioxidative activities based on reducing power.
Keywords:
ultra-fine Ulmus davidiana var. japonica, antioxidant activity, phenolic compoundsI. Introduction
Ulmus davidiana (U. davidiana) is a traditionally used in Korean medicine with anticancer, antiviral, antibacterial, and anti-inflammatory properties (Song et al. 2007). It is widely cultivated in Asia including Korea and its bark of the stem and roots are used extensively (Jung et al. 2008). Dried and grounded inner bark into powder is used in soups or cereal flours as a thickening agent in food manufacturing (Jung et al. 2008). Catechin and related compounds were detected as phytochemicals of U. davidiana stem bark (Jung et al. 2008).
Several oxygen species with high reactivity (ROS), such as superoxide anion (O.-), hydrogen peroxide (H2O2), and hydroxyl radical (.OH), are nearly related to incidence of Alzheimer’s disease, aging, cancer, infection, and cardiovascular disease (Freeman 1984; Squadrito & Pryor 1998). The interest has been raised in searching antioxidant phytochemicals, because those compounds may hinder the process of free radical reactions (Kinsella et al. 1993). These components are regarded as effective free radical scavengers and inhibitor of lipid oxidation due to their metal chelating properties (Miller 1997). The most effective agent appears to be herbs, seeds, and fruits in most of plants.
An antioxidant is defined that delays or inhibits the oxidation process with low concentration (Halliwell 1995). The antioxidants such as ascorbic acid, carotenoids, flavonoids and tannins, are considered to play an important role in inhibiting induced disease by free radicals (Halliwell et al. 1992). In addition, an inverse relationship was found between antioxidant-rich food consumption and disease incidence rate (Rice-Evans et al. 1997; Kim et al. 2014).
In recent, butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) as synthetic antioxidants are widely used in processed foods and there is possibility showing side effects (Ahn & Park 2013). However, potent antioxidant activities without any side effect were found in plant as medicinal property in recent studies. By using these medicinal components could be established as natural medicines for prevention and treatment of related diseases ( Jung et al. 2008; Choudhary & Swarnkar 2011).
Several previous studies have indicated that water and various solvent extracts of U. davidiana exhibited a high antioxidant property (Guo & Wang 2007; Jung et al. 2008; Ahn & Park 2010; Park 2011). Also, the change of several aspects such as physicochemical and microbial properties of U. davidiana powder was investigated during storage (Ahn 2014). However, there was no information on antioxidant activity of ultra-fine U. davidiana particles, even though ultra-fine sizing of various plants increase the functional value (Seo et al. 2011; Ahn et al. 2013; Ahn et al. 2014). Therefore, the present study was designed to study the antioxidant properties of ultra-fine powdered U. davidiana stem barks.
Ⅱ. Materials and Methods
1. Materials
Commercial bark of stem powder of Ulmus davidiana (U. davidiana) var. japonica, which was dried under room temperature was purchased. Ultra-fine U. davidiana powder (UFP) was manufactured by the dry milling in Apexcel Co. (Pohang, Korea) and stored at room temperature. The average particle size of UFP was in the range of 1-3 μm and commercial powder (CP) was about 100 μm. All chemicals and reagents were purchased from Sigma Chemicals Co. (St. Louis, MO, USA).
2. Preparation of extracts
For ethanol (EtOH) extraction, three hundred grams of either CP or UFP were dissolved in 1,000 mL of EtOH after 24 hr soaking. Then, soluble collected EtOH fraction was concentrated to about 20 mL volume using a vacuum evaporator and kept in a refrigerator until use. For hot-water extraction, 300 g of either CP or UFP U. davidiana var. japonica were boiled in 1,000 mL for 2 hr, filtered and concentrated with freeze-dryer.
3. DPPH radical scavenging activity
The antioxidant activity was determined with its ability in stabilizing the free radical DPPH (1,1-diphenyl-2-picrylhydrazyl). The mixture containing 40 μL of extracts (4 mg/mL dissolved in dimethyl sulfoxide (DMSO)) and 760 μL of 300 μM DPPH solution was heated at 37℃ for 30 min and the absorbance was determined at 515 nm. The DPPH ability was calculated and all determination was triplicated.
DPPH radical scavenging activity (%) = [(A0-A1)/A0]x100,
where A0 was the absorbance of the control and A1 was the absorbance of the sample.
4. ABTS radical scavenging activity
ABTS (2,2-Azino-bis-3-ethylbenzothiazoline-6-sulfonic acid) radical scavenger ability was measured with modification (Re et al. 1999). Seven mM of ABTS reagent was mixed with 2.45 mM potassium persulfate and stood for 12-16 hr in dark. After ABTS radical was produced, it was diluted upto 0.70 absorbance value at 734 nm. The 40 uL of sample (4 mg/mL dissolved in DMSO) and 760 uL ABTS radical solution were mixed and incubated at room temperature for 10 min. At 734 nm, the absorbance was measured and the ABTS ability was calculated. All measurements were in triplicate.
ABTS radical scavenging activity (%) = [(A0-A1)/A0]x100,
where A0 was the absorbance of the control and A1 was the absorbance of sample.
5. Hydroxyl radical scavenging activity
Hydroxyl radical scavenger ability was measured with a few modification (Smirnoff & Cumbes 1989). The mixture contained 250 μL of FeSO4 (1.5 mM), 175 μL of hydrogen peroxide (6 mM) and 300 μL of sodium salicylate (20 mM) and reacted for 30 min at 37℃. The absorbance was determined at 562 nm and activity was calculated. All determination was triplicated.
6. Reducing power
The reducing power was examined by the method of Oyaizu(1986). Different concentrations of 2.5 mL of each samples were reacted with 2.5 mL (200 mM) of sodium phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide. After 20 min incubation at 50℃, 2.5 mL of 10% trichloroacetic acid (w/v) were added. Then, the mixture was centrifuged at 650 rpm for 10 min, and the upper layer (5 mL) was collected. Five mL of deionized water and 1 mL of 0.1% of ferric chloride were added and the absorbance was measured at 700 nm.
7. Phenolic compound
One mg of the sample was mixed with 2 mL of Foiln-Denis reagent and 2 mL of 35% sodium carbonate, mixed vigorously and filled up to 10 mL with distilled water. After 30 min incubation at ambient temperature, the absorbance was measured at 765 nm, The contents of phenolic compound were determined through a standard curve prepared at different concentrations of tannic acid.
8. Statistical analysis
Statistical analysis system (version 9.0, SAS Institute Inc. Cary, NC, USA) was used. ANOVA was used to test significant differences among the samples, and means were performed by Duncan’s multiple range test (p<0.05).
III. Results and Discussion
1. Phenolic contents
The total phenolic contents of either CP or UFP U. davidiana ver. japonica were shown in Table 1. The phenolic content of CP and UFP extraction samples in EtOH were 40.38 and 65.61 mg tannic acid equivalent/g, respectively. However, a lower content of phenolic compound was found in hotwater such as 39.68 mg tannic acid equivalent/g in CP and 45.62 mg tannic acid equivalent/g in UFP U. davidiana var. japonica.
Earlier study showed that phenolic compounds have been shown to possess a high antioxidant property (Guo & Wang 2007). Other previous study have also shown a colse relationship between total phenolic content and an antioxidant capacity in some berry crops (Kalt et al. 1999).
Polyphenols belong to a several kinds of materials with a high antioxidant actions. Those compounds are generally known to stabilize oxygen-derived free radicals by contributing a hydrogen atom to the free radical (Guo & Wang 2007). In searching for sources of natural antioxidants, the radical scavenging activities of medicinal plants and fruits have been studied in the last few decades (Guo & Wang 2007).
2. DPPH radical scavenging activity
The antioxidant activity of DPPH is thought to be due to their hydrogen-donating ability. In this study, scavenging activity of EtOH or hot-water extract on DPPH was compared between CP and UFP U. davidiana var. japonica and shown in Fig. 1.
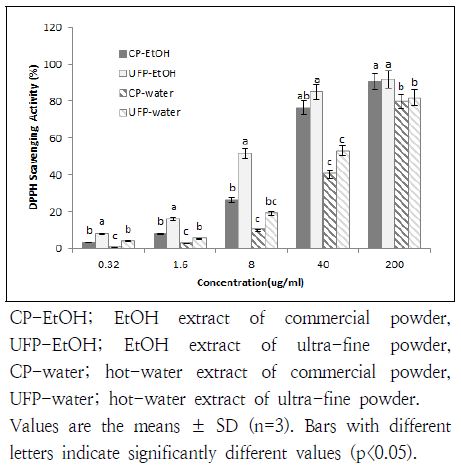
DPPH radical scavenging activities of commercial or ultra-fine Ulmus davidiana powder at different concentrations
DPPH radical scavenging activity increased with increasing extract concentrations (Fig. 1). At every concentrations, UFP-EtOH showed the highest activity among other samples. Also, the DPPH scavenging activity was significantly higher at lower concentrations (0.32, 1.6, and 8 μg/mL) (Fig. 1). The activity reached up to 81.8 and 76.7% at 200 μg/mL concentration in UFP-water and CP-water, which were significantly lower activities compared with those in EtOH extracts (90.4 and 91.8%). DPPH radical scavenging activity of water extract was decreased dramatically at 40 μg/mL concentration.
Both CP-EtOH and UFP-EtOH showed a strong antioxidant activity (IC50 at 12.56 and 6.75 μg/mL), whereas IC50 which reflects 50% depletion of DPPH free radicals in CP-water and UFP-water were 70.05 and 31.64 μg/mL (data not shown). These data were similar to those of other study, which evaluated the free radical scavenging activity of U. davidiana extracts with various solvents (Guo & Wang 2007). A high positive correlation has been found between total polyphenolic contents and DPPH radical scavenging ability (Guo & Wang 2007).
Therefore, the present study indicated that EtOH extract showed the highest antioxidative activity compared with other samples, therefore, EtOH extract may act as free radical inhibitors or scavengers.
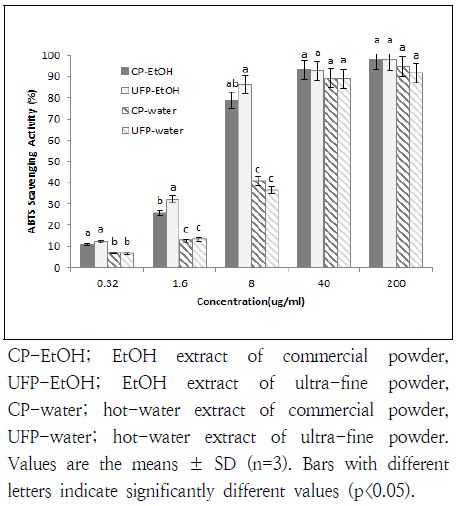
3. ABTS radical scavenging activity
Scavenging activity of EtOH or hot-water extracts on ABTS was compared between CP and UFP U. davidiana var. japonica and shown in Fig. 2. There was no difference in samples at 40 and 200 μg/mL concentrations (p<0.05). At 200 μg/mL, scavenging activities were found in the range of 91.6 to 98.2%, and over 89% of scavenging activity was shown in all samples at 40 μg/mL concentration. The IC50 values was 3.76 and 2.96 μg/mL of CP-EtOH and UFP-EtOH (data not shown) and UFP and CP in hot-water extraction showed the similar scavenging activity with IC50 (8.62 and 9.48 μg/mL, respectively). However, extracts of hotwater showed the significantly lower activity than that of EtOH at 8 μg/mL and lower concentrations (p<0.05). These results indicated that both UFP and CP extracts of U. davidiana var. japonica showed a strong antioxidative activity on ABTS at high concentration, regardless of EtOH and hot-water extraction.
4. Hydroxyl radical scavenging activity
In hydroxyl radical scavenging assay, both activities of CP and UFP in EtOH extraction increased moderately up to 73.5 and 67.9% at 200 μg/mL (Fig. 3). There was no dramatic difference between samples. Hot-water extracts showed slightly lower activities at 40 and 200 μg/ml concentration than EtOH extracts. Also, not much difference was found between CP and UFP in hydroxyl scavenging activity in both EtOH and hot-water extraction.
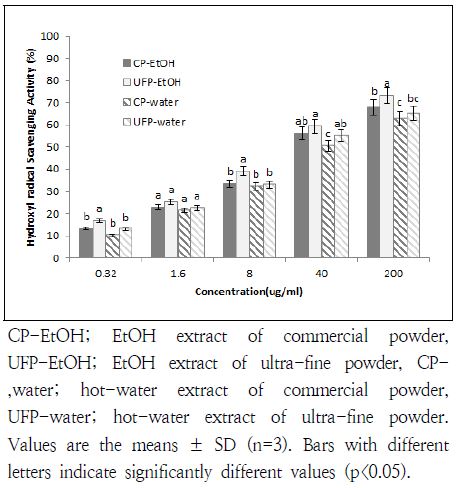
Hydroxyl radical scavenging activities of commercial or ultra-fine Ulmus davidiana powder at different concentrations
Studies of effective hydroxyl radical scavengers indicate that the activity is closely related to the phenolic contents (Guo & Wang 2007). Phenolic compounds are widely contained in both edible and inedible plants, and those have been known to show various functional effects (Guo & Wang 2007). These antioxidant activity is mostly due to their redox properties, which can be an aspects in absorbing free radicals (Siriwardhana et al. 2003). Therefore, a slightly higher activity found in EtOH extracts of UFP is likely due to higher contents of phenolic compound in samples.
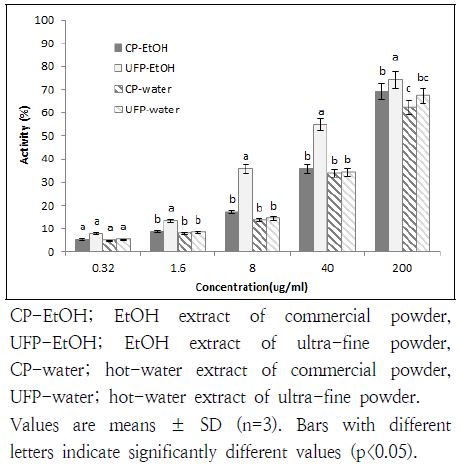
5. Reducing power
The reducing power may be a significant indicator of its potential antioxidant ability (Jung et al. 2008). Fig. 4 presents the reductive capabilities of both CP and UFP U. davidiana var. japonica on EtOH and hot-water. The significantly high reducing power was shown in UFP-EtOH group, compared with others at every concentrations except for 0.32 μg/mL (p<0.05).
Reducing power is closely related to the reductones, which have been known as an antioxidant agent by giving a hydrogen atom (Duh 1998). In our study, EtOH extracts of UFP showed a higher reducing activity, which suggests that there may be some reductones contained in UFP U. davidiana var. japonica.
In present, BHA and BHT, the synthetic antioxidants, are commomly used to reduce lipid peroxidation and to prolong the shelf-life of food products. Recent studies have been focused on natural compounds as a natural antioxidants derived from medicinal plants. In consequence, it is widely accepted that some of synthetic antioxidants need to be replaced because of their potential health risk (Li et al. 2008).
Several previous studies have indicated that U. davidiana extracts of various solvents showed a strong antioxidant activity (Guo & Wang 2007; Jung et al. 2008; Ahn & Park 2010; Park 2011). However, no study has been done to evaluate the effect of ultra-fine size for extraction process. The results of the various inhibitor assays revealed that EtOH extract of U. davidiana var. japonica could be considered as an effective additive in food and pharmaceutical agent.
IV. Summary and Conclusion
The present study was designed to compare the antioxidant activities between commercially powdered (CP) and ultra-fine powdered (UFP) Ulmus davidiana (U. davidiana) var. japonica. The extraction was performed using either ethanol (EtOH) or hotwater. The phenolic content of CP and UFP U. davidiana extracts in EtOH were 40.38 and 65.61 mg tannic acid equivalent/g. In DPPH, EtOH extracts showed a significantly higher activity than hot-water extracts at all concentrations (p<0.05). The reducing power of UFP U. davidiana var. japonica in EtOH extraction exhibited 74.3%, which was the significantly higher value than other samples (p<0.05). In addition, reducing powder was significantly higher in UFP-EtOH than those of others at every concentrations except for 0.32 μ g/mL (p<0.05).
In present, synthetic antioxidants are commonly used in food manufacturing for prevention of oil oxidation and extension of food shelf-life, while some synthetic antioxidants may possess potential health risks. The present data indicated that ultra-fine powder of U. davidiana extracts show higher antioxidative activities such DPPH radical scavenging activity and reducing power, therefore, could be used as potential source of antioxidant materials. In the health view point, U. davidiana var. japonica powder could be considered as an effective additive in food and pharmaceutical agent.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education, Science and Technology(2011-0013614).
References
-
Ahn, J., (2014), Physicochemical, microbial, and sensory properties of yogurt with Ulmus davidiana var. japonica during storage, Korean J Community Living Sci, 25(4), p610-608.
[https://doi.org/10.7856/kjcls.2014.25.4.601]

-
Ahn, J., Lee, JS., Yang, KM., (2014), Ultrafine particles of Ulmus davidiana var. japonica induce apoptosis of gastric cancer cells via activation of caspase and endoplasmic reticulum stress, Arch Pharm Res, 37(6), p783-793.
[https://doi.org/10.1007/s12272-013-0312-2]

- Ahn, J., Park, JH., (2010), Antioxidant activity and protective effect on DNA damage of extracts from Ulmus davidiana var. japonica, J Appl Oriental Med, 10(2), p9-16.
-
Ahn, J., Park, JH., (2013), Effects of Abeliophyllum distichum Nakai flower extracts on antioxidative activities and inhibition of DNA damage, Korean J Plant Res, 26(3), p355-361.
[https://doi.org/10.7732/kjpr.2013.26.3.355]

-
Ahn, YJ., Ganesan, P., Yoo, SH., Kwak, HS., (2013), Effect of nanopowdered peanut sprouts supplementation on physicochemical and sensory properties of milk, Korean J Food Sci An, 33(1), p9-15.
[https://doi.org/10.5851/kosfa.2013.33.1.9]

-
Duh, PD., (1998), Antioxidant activity of burdock (Arctium lappa Linne): its scavenging effect on free radical and active oxygen, J Am Oil Chem Soc, 75(4), p455-461.
[https://doi.org/10.1007/s11746-998-0248-8]

- Freeman, BA., (1984), Biological sites and mechanism of free radical production, In: Armstrong, D., Sohal, R., Culter, RG., Slater, T. eds., New York, Free Radicals in Molecular Biology, Aging, and Disease, p43-52.
- Guo, J., Wang, MH., (2007), Antioxidant and antidiabetic activities of Ulmus davidiana Extracts, Food Sci Biotechnol, 16(1), p55-67.
- Halliwell, B., Gutteridge, JMC., Cross, CE., (1992), Free radicals, antioxidants, and human disease: Where are we now?, J Lab Clin Med, 119(6), p598-620.
-
Halliwell, B., (1995), Antioxidant characterization: methodology and mechanism, Biochem Pharmacol, 49(10), p1341-1348.
[https://doi.org/10.1016/0006-2952(95)00088-H]

-
Jung, H., Kang, H., Song, YS., Lim, C., Park, E., (2008), Antioxidant and anti-nociceptive activities of Ulmus davidiana var. japonica, Biomol Therapeu, 16(1), p9-13.
[https://doi.org/10.4062/biomolther.2008.16.1.009]

-
Kalt, W., Fomey, CF., Martin, A., Prior, RL., (1999), Antioxidant capacity, vitamin C, phenolics, and antocyanins after fresh storage of small fruits, J Agri Food Chem, 47(11), p4638-4644.
[https://doi.org/10.1021/jf990266t]

-
Kim, SY., Lee, YM., Kim, JB., Park, DS., Go, JS., Kim, HR., (2014), Comparison of phytochemical properties and antioxidant activity between raw and heat-treated vegetables, Korean J Community Living Sci, 25(1), p5-18.
[https://doi.org/10.7856/kjcls.2014.25.1.5]

- Kinsella, JE., Frankel, E., German, B., Kanner, J., (1993), Possible mechanisms for the protective role of antioxidants in wine and plant foods, Food Technol, 47(1), p85-89.
-
Li, HB., Wong, CC., Cheng, KW., Chen, F., (2008), Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants, Food Sci Technol LEB, 41(3), p385-390.
[https://doi.org/10.1016/j.lwt.2007.03.011]

- Miller, NJ., (1997), Flavonoids and phenylpropanoids as contributors to the antioxidant activity of fruit juices, In Rice-Evans, CA., Packer, L. eds., New York, Flavonoids in Health and Disease, p387-405.
-
Oyaizu, M., (1986), Studies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine, Jpn J Nutr, 44(6), p307-315.
[https://doi.org/10.5264/eiyogakuzashi.44.307]

- Park, JH., (2011), Antioxidant activities and inhibitory effect on oxidative DNA damage of extracts from Abeliophyllum distichum folium, Korean J Herbiol, 26(4), p95-99.
-
Re, P., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., Evans, C., (1999), Antioxidant activity applying an improved ABTS radical cation decolorization assay, Free Radical Bio Med, 26(9-10), p1231-1237.
[https://doi.org/10.1016/S0891-5849(98)00315-3]

-
Rice-Evans, CA., Sampson, J., Bramley, PM., Hollowa, DE., (1997), Why do we expect carotenoids to be antioxidants in vivo, Free Radic Res, 26(4), p381-398.
[https://doi.org/10.3109/10715769709097818]

-
Seo, MH., Lee, SY., Chang, YH., Kwak, HS., (2011), Physicochemical, microbial, and sensory properties of milk supplemented with nanopowdered chitosan during storage, J Dairy Sci, 92(12), p5907-5916.
[https://doi.org/10.3168/jds.2009-2520]

-
Siriwardhana, N., Lee, KW., Kim, SW., Ha, WJ., Jeon, YJ., (2003), Antioxidant activity of Hizikia fusiformis on reactive oxygen species scavenging and lipid peroxidation inhibition, Food Sci Technol Int, 9(5), p339-346.
[https://doi.org/10.1177/1082013203039014]

-
Smirnoff, N., Cumbes, QJ., (1989), Hydroxyl radical scavenging activity of compatible solutes, Phytochem, 28(5), p1057-1060.
[https://doi.org/10.1016/0031-9422(89)80182-7]

-
Song, I., Kim, K., Suh, S., Kim, M., Kwon, DY., Kim, S., Kim, C., (2007), Anti-flammatory effect of Ulmus davidiana planch (Ulmaceae) on collagen-induced inflammation in rats, Environ Toxicol Pharmacol, 23(1), p102-110.
[https://doi.org/10.1016/j.etap.2006.07.013]

-
Squadrito, GL., Pryor, WA., (1998), Oxidative chemistry of nitric oxide. The roles of superoxide, peroxynitrite, and carbon dioxide, Free Radical Biol Med, 25(4-5), p392-403.
[https://doi.org/10.1016/S0891-5849(98)00095-1]